Abstract
Background: In Chuvash polycythemia (CP), homozygous germline VHLR200W results in augmented hypoxia-sensing, elevated erythropoietin and hemoglobin, and increased morbidity and mortality from venous and arterial thromboses.1 In CP patients and controls from Russia's Chuvash Republic, the probability of new thrombosis in CP increased with age, smoking, and higher expression of THBS1, the gene encoding thrombospondin-1.2 In mice, hypoxia leads to upregulation of THBS1 through HIF-23 and to upregulation of VWF .4 THBS1 reportedly regulates the size of circulating von Willebrand Factor (VWF) multimers5 through inhibiting proteolytic cleavage of VWF by ADAMTS13.6 We hypothesized that upregulation of THBS1 in CP may be a risk for thrombosis through an effect on ADAMTS13 and VWF.
Methods: We enrolled into an observational study 155 patients with CP and 154 controls matched by age, sex and place of residence. Written informed consent was obtained from all subjects according to the Declaration of Helsinki. We genotyped for VHLR200W, measured baseline plasma THBS-1 concentrations2 and followed the subjects for a median of 9.4 years. Here, we report results for determining baseline circulating levels of ADAMTS13 antigen, activity and specific activity and of VWF antigen and differential multimers in up to 57 CP patients and 30 controls.
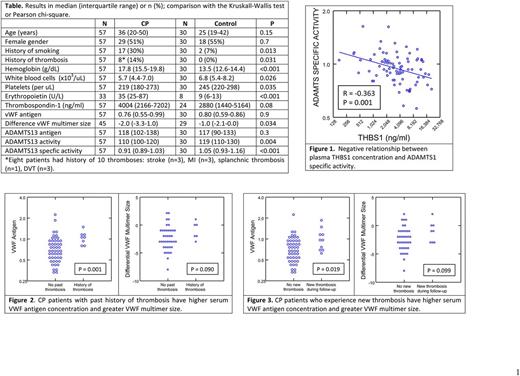
Results: CP subjects had higher hemoglobin and erythropoietin concentrations and lower white blood cell and platelet counts (Table 1). They were also more likely to be current smokers. There was a trend to higher THBS-1 concentrations in the CP patients (p=0.084), which was significant after adjusting for platelet count (P=0.016). ADAMTS13 and VWF antigen levels did not differ between CP patients and controls but ADAMTS13 activity and especially specific activity were lower in CP. Plasma THBS-1 concentration correlated positively with ADAMTS13 antigen concentration (r=0.358, P=0.001) but negatively with specific activity (r=-0.363, P=0.001) (Figure 1). Eight (14.0%) CP patients (aged 27-76) years had a history of thrombosis (Table 1). The mean serum VWF antigen concentration (P=0.001) and differential VWF multimer size (P=0.090) were higher in CP patients with a history of thrombosis (Figure 2). During the prospective follow-up period, 10 CP patients (17.5%) experienced a new thrombosis at a median age of 46 (range 26-70) years. Three patients also experienced an additional thrombosis for a total of 13 new thromboses: myocardial infarction (n=5), stroke (n=3), splanchnic thrombosis (n=2), deep vein thrombosis (n=2), pulmonary embolism (n=1). Baseline serum VWF antigen levels (P=0.019) and VWF multimer size (P=0.099) were higher in subjects who developed a new thrombosis during follow-up (Figure 3), and these relationships persisted after adjusting for smoking history.
Discussion: THBS1 is an adhesive glycoprotein that mediates cell-to-cell and cell-to-matrix interactions and facilitates platelet aggregation.7 In mice, platelet-derived THBS1 modulated arterial thrombosis only in the presence of VWF.8 Our present findings suggest that CP subjects have lower ADAMTS13 specific activity than controls and that this lower activity may be related to higher plasma THBS1 concentrations. Lower ADAMTS13 specific activity suggests the presence of an inhibitor or partial inactivation of the protease. Furthermore, higher VWF antigen and multimer size in CP patients is associated with a past history of thrombosis and a greater risk for new thrombosis in the future. More broadly, our findings suggest a possible implication of VWF in thrombosis of conditions associated with an up-regulated hypoxic response. Further studies may identify new pathways of prevention and treatment with broad applicability.
References
1. Ang SO, Chen H, Hirota K, et al. Nat Genet 2002;32:614-21.
2. Sergueeva A, Miasnikova G, Shah BN, et al. Haematologica 2017.
3. Labrousse-Arias D, Castillo-Gonzalez R, Rogers NM, et al. Cardiovasc Res 2016;109:115-30.
4. Singh B, Biswas I, Bhagat S, Surya Kumari S, Khan GA. Eur J Immunol 2016;46:2388-400.
5. Pimanda JE, Ganderton T, Maekawa A, et al. J Biol Chem 2004;279:21439-48.
6. Wang A, Liu F, Dong N, et al. Thromb Res 2010;126:e260-5.
7. Isenberg JS, Romeo MJ, Yu C, et al. Blood 2008;111:613-23.
8. Prakash P, Kulkarni PP, Chauhan AK. Blood 2015;125:399-406.
Gordeuk: Emmaus Life Sciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal